Covalent Bond vs Metallic Bond
The chemical bonds are stable attractions between atoms, ions or molecules. The formation of chemical bond allows the formation of molecules or compounds. Chemical bonds are classified into different categories based on their formation and strength. Important types of chemical bonds recognized are Covalent bonds, Ionic bonds, Metallic bonds, Dipole-dipole interactions, London dispersion forces and Hydrogen bonds.
The present post discuss about the Similarities and Differences between the Covalent bond and Metallic bond with a Comparison Table.
| You may also like NOTES in... | ||
|---|---|---|
| BOTANY | BIOCHEMISTRY | MOL. BIOLOGY |
| ZOOLOGY | MICROBIOLOGY | BIOSTATISTICS |
| ECOLOGY | IMMUNOLOGY | BIOTECHNOLOGY |
| GENETICS | EMBRYOLOGY | PHYSIOLOGY |
| EVOLUTION | BIOPHYSICS | BIOINFORMATICS |
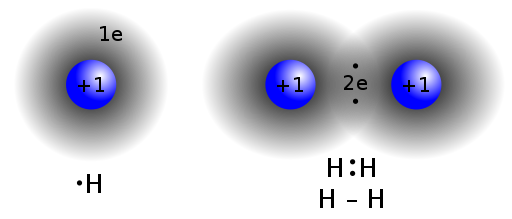
Covalent bond: A chemical bond formed by the sharing of electron pairs.
Metallic bond: Bonds occurs in metals, formed by the electrostatic attractive force between delocalized electrons and positively charged metal ions.
Similarities between Covalent Bond and Metallic Bond
Ø Both covalent and metallic bonds are strong bonds.
Ø Both are primary bonds.
Ø Both bonds result in the formation of complex structures.
Ø The formation of both covalent and metallic bond results in the formation of more stable compounds than the original.
Ø Compounds with covalent and metallic bonds are insoluble in polar solvents.
Difference between Covalent Bond and Metallic Bond