What are Disaccharides?
Disaccharides are carbohydrates which contain two covalently linked monosaccharide units. Sucrose, Maltose, Lactose, Trehalose and Cellobiose are naturally occurring disaccharides. The individual monosaccharide units in a disaccharide are called ‘residues’. All disaccharides are soluble in water
Glycosidic bonds links monosaccharide units
The monosaccharide units in disaccharides (and also in polysaccharides) are linked through a special type of covalent bond called Glycosidic bond (specifically O-glycosidic bond). O-glycosidic bond is formed by the reaction between the hydroxyl group of one monosaccharide with the anomeric carbon atom of the other. During the glycosidic bond formation, one molecule of water is eliminated as given in the diagram. Glycosidic bonds are strong covalent bonds and they can be hydrolyzed by treating with mild acids. The hydrolysis of the glycosidic bond of a disaccharide releases its corresponding monosaccharide units.

More in Biochemistry: Lecture Notes, MCQ, PPTs, Videos
Some disaccharides are reducing sugars some are NOT:
Some disaccharides are reducing sugars while some others are non-reducing. If the anomeric carbon atom of both the monosaccharide residues in the disaccharide is involved in the glycosidic bond formation, then the disaccharides are unable to reduce the ferric or cupric ions and thus they will be non-reducing sugars. Example: Sucrose and Trehalose.
| You may also like NOTES in... | ||
|---|---|---|
| BOTANY | BIOCHEMISTRY | MOL. BIOLOGY |
| ZOOLOGY | MICROBIOLOGY | BIOSTATISTICS |
| ECOLOGY | IMMUNOLOGY | BIOTECHNOLOGY |
| GENETICS | EMBRYOLOGY | PHYSIOLOGY |
| EVOLUTION | BIOPHYSICS | BIOINFORMATICS |
If at least one anomeric carbon is free in a disaccharide, it can reduce the ferric or cupric ions and thus they will be reducing sugars. Example: Lactose, Maltose and Cellobiose.
Homodisaccharides vs Heterodisaccharides
Based on the composition, the disaccharides are divided into homodisaccharides and heterodisaccharides. In homodisaccharides, both the monosaccharide units are the same. Example: Maltose, Isomaltose, and Cellobiose. In heterodisaccharide, the monosaccharide units are different. Example – Sucrose (a disaccharide of glucose and fructose) and lactose (a disaccharide of galactose and glucose).
Examples of Disaccharides: Sucrose, Lactose, Maltose, Trehalose and Cellobiose
(1). Sucrose
Ø Sucrose is also called as ‘Table Sugar’.
Ø It is a disaccharide of Glucose and Fructose joined by α-1-β2-glycosidic linkage.
Ø C1 of the glucose is bonded with the C2 of fructose (both are anomeric carbons).
Ø The chemical formula of sucrose is C12H22O11.
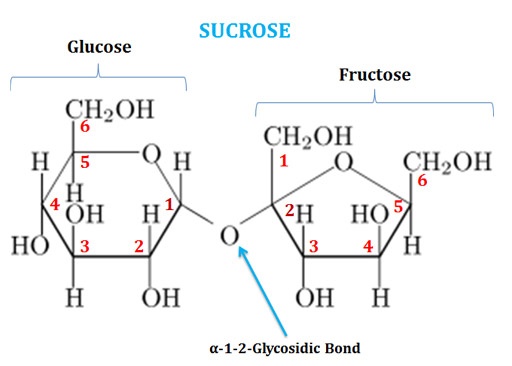
Ø The systematic name of sucrose is O-α-D-glucopyranosyl-(1→2)-β-D-fructofuranoside.
Ø In sucrose, the glucose residue is in six-membered ring form (Pyranose form) and the fructose residue is in five-membered ring form (Furanose form).
Ø Sucrose is a non-reducing sugar since the anomeric carbon atoms of both glucose and fructose are involved in glycosidic bond formation.
Ø Sucrose is formed only in plants. No animals are known to produce sucrose.
Ø Sucrose is the major intermediate product of photosynthesis.
Ø It is the major form of sugar transport in plants.
Ø Sucrose is hydrolyzed by the enzyme Invertase into glucose and fructose.
(2). Lactose
Ø Lactose is the milk sugar, present naturally only in milk.
Ø In milk, the concentration of lactose varies from 2 – 8% in different species.

Ø It is a disaccharide of Galactose and Glucose connected by β(1-4) glycosidic linkage.
Ø The systematic name of lactose is O-β-D-galactopyranosyl-(1→4)-D-glucopyranose.
Ø The chemical formula of lactose is C12H22O11.
Ø In lactose, the anomeric carbon on the glucose is free and thus it a reducing sugar.
Ø Lactose cannot be absorbed directly into the bloodstream of animals.
Ø First, it should be hydrolyzed into its monomer units (galactose and glucose) by the activity of the enzyme β-Galactosidase commonly called as lactase.
Ø In mammals, the lactase enzyme is produced only by young milk feeding individuals.
Ø As the individuals mature the production of lactase enzyme decreases.
Ø Most adult humans, except certain groups in Africa and northern Europe, produce only very low levels of lactase.
Ø Thus, the adults cannot utilize lactose as a food source. For most individuals, this is not a problem, but some cannot tolerate lactose and experience intestinal pain and diarrhea upon consumption of milk.
Ø This condition is called Lactose Intolerance.
Ø The lactose intolerance is caused by the fermentation of lactose inside the colon of adult individuals by some gut bacteria.
Ø Lactase fermentation in the colon produced CO2, H2, and some irritating organic acids.
Ø These compounds cause the irritation, painful stomach upset and diarrhea.
(3). Maltose
Ø Maltose is also called as malt sugar or maltobiose.
Ø It is a disaccharide of two glucose residues connected through α(1-4) glycosidic linkage.
Ø Maltose possesses the free anomeric carbon atom at the second glucose and thus it is a reducing sugar.
Ø Maltose is produced from starch by the activity of the enzyme β-amylase.

Ø β-amylase enzyme produces two-unit groups from the starch.
Ø Maltose is the two-unit member of the amylose part of starch.
Ø Maltose is the major component of the ‘Malt’.
Ø The ‘Malt’ is formed when starchy grains are allowed to germinate by soaking in water.
Ø An enzyme called diastase, produced during the germination of seeds catalyzes the hydrolysis of starch to maltose.
Ø Maltose is used in beverages industry for the production of beer by yeast fermentation.
(4). Trehalose
Ø Trehalose (also called mycose) is a disaccharide of two glucose residues connected through α(1-1)-glycosidic linkage.
Ø The systematic name of trehalose is α-D-glucopyranosyl-(1→1)-α-D-glucopyranoside.
Ø In trehalose, both the anomeric carbon atoms are involved in glycosidic bond formation. Thus, it is a non-reducing sugar.
Ø Trehalose is the major constituent of hemolymph of insects.
Ø Trehalose can be synthesized by bacteria, fungi, plants and some invertebrates.
Ø Trehalose helps plants to survive during desiccations.

| You may also like... | ||
|---|---|---|
| NOTES | QUESTION BANK | COMPETITIVE EXAMS. |
| PPTs | UNIVERSITY EXAMS | DIFFERENCE BETWEEN.. |
| MCQs | PLUS ONE BIOLOGY | NEWS & JOBS |
| MOCK TESTS | PLUS TWO BIOLOGY | PRACTICAL |
(5). Cellobiose
Ø Cellobiose is a disaccharide of two glucose molecules connected through β(1→4) glycosidic linkage.
Ø It is a reducing sugar since the anomeric carbon atom of second glucose is free.

You might also like…
@. Carbohydrates Part 1: Introduction
@. Carbohydrates Part 2: Monosaccharides
@. Carbohydrates Part 3: Disaccharides
@. Carbohydrates Part 4: Polysaccharides
@. Carbohydrates Part 5: Glycoconjugates (Glycoproteins & Proteoglycans)
@. Carbohydrates Part 6: Glycosaminoglycans
@. Membrane Lipids: Properties, Structure and Classification
@. MCQ on Carbohydrates: Part 1 | Part 2 | Part 3 |
More Lecture Notes from Easy Biology Class…
BotanyZoologyBiochemistryGeneticsMolecular BiologyBiotechnologyEndocrinologyPhysiologyMicrobiologyImmunologyEmbryologyEcologyEvolutionBiophysicsResearch MethodologyBiostatisticsChemistry for BiologistsPhysics for Biologists
Browse more in Easy Biology Class…
Lecture NotesBiology PPTsVideo TutorialsBiology MCQQuestion BankDifference betweenPractical AidsMock Tests (Online)Biology Exams

Please upload polysaccharide notes🙏 all notes are very helpful
Are polysaccharide notes ready?
Sir why polyasccharide is not open…?
Will be updated soon