Introduction
Have you ever wondered how doctors can detect specific proteins in your blood, such as those produced by pathogens or even by your own body in response to disease? One powerful technique they use is called ELISA, which stands for Enzyme-Linked Immunosorbent Assay. In this blog post on ELISA short notes, we’ll break down ELISA technique into simple terms, show you how it works, and highlight some examples. You can download the PDF of this notes from the download link provided below the post.
What is ELISA?
ELISA is a laboratory technique used to detect the presence of an antibody or an antigen (a substance that triggers an immune response) in a sample. It is a super-sensitive molecular detective technique can help to diagnose diseases, check for allergies, and even monitor hormone levels.
Ø First described by Eva Engvall and Peter Perlmann in 1971
Ø ELISA is a solid-phase type of enzyme immunoassay (EIA) to detect the presence of a ligand (commonly a protein) in a liquid sample using antibodies directed against the ligand to be measured.
Ø ELISA has been used as a diagnostic tool in medicine, plant pathology, and biotechnology, as well as a quality control check in various industries.

How Does ELISA Work?
Think of ELISA as a lock-and-key system where specific antibodies (the keys) bind to their matching antigens (the locks). Here’s a step-by-step breakdown of ELISA:
- Coating: The test begins with a plate (usually made of plastic) that has wells coated with either the antigen or the antibody, depending on what you’re testing for.
- Blocking: To prevent non-specific binding, the wells are blocked with a protein solution that fills in all the spaces where the target molecule isn’t supposed to bind.
- Adding the Sample: The sample (e.g., blood, urine) is added to the wells. If the target antigen or antibody is present in the sample, it will bind to the coating on the wells.
- Detection Antibody: A detection antibody, which is specific to the antigen or antibody of interest, is added. This detection antibody is conjugated (linked) to an enzyme or can itself be detected by a secondary antibody that is linked to an enzyme through bioconjugation.
- Wash: The unbound antibodies or antigens are washed out from the well using suitable wash buffers.
- Substrate Addition: A substrate is added to the wells. The enzyme on the detection antibody reacts with the substrate, producing a color change.
- Reading the Results: The intensity of the color change is measured using a spectrophotometer. The amount of color change is proportional to the amount of antigen or antibody in the sample.
| You may also like NOTES in... | ||
|---|---|---|
| BOTANY | BIOCHEMISTRY | MOL. BIOLOGY |
| ZOOLOGY | MICROBIOLOGY | BIOSTATISTICS |
| ECOLOGY | IMMUNOLOGY | BIOTECHNOLOGY |
| GENETICS | EMBRYOLOGY | PHYSIOLOGY |
| EVOLUTION | BIOPHYSICS | BIOINFORMATICS |
Types of ELISA
There are several types of ELISA, each used for different purposes: (1) Direct ELISA, (2) Sandwich ELISA
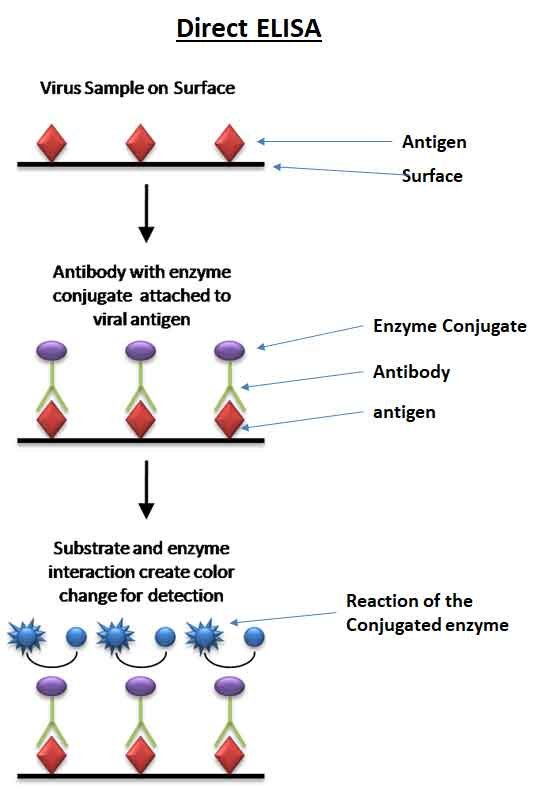
Direct ELISA
The antigen is directly attached to the plate and detected with an enzyme-linked antibody.
The steps of direct ELISA follow the mechanism below:
- A buffered solution containing the antigen to be tested is added to each well of a microtiter plate, allowing it time to adhere to the plastic through charge interactions.
- A solution of nonreacting protein, such as bovine serum albumin or casein, is added to each well to cover any plastic surface that remains uncoated by the antigen.
- The primary antibody with an attached enzyme is added, which binds specifically to the antigen coating the well.
- A substrate for this enzyme is then added, often changing color upon reaction with the enzyme.
- The higher the concentration of the primary antibody in the serum, the stronger the color change, which is often measured quantitatively using a spectrometer.
Ø The enzyme acts as an amplifier; even if few enzyme-linked antibodies remain bound, the enzyme will produce many signal molecules. While the enzyme can produce color indefinitely within reasonable limits, a higher antibody concentration results in faster color development.
Ø The disadvantage of direct ELISA is the non-specific method of antigen immobilization; when serum is used as the test antigen source, all proteins in the sample may adhere to the microtiter plate, causing small analyte concentrations in serum to compete with other serum proteins for binding to the well surface.
Ø The sandwich or indirect ELISA addresses this issue by using a “capture” antibody specific to the test antigen, isolating it from the serum’s molecular mixture.
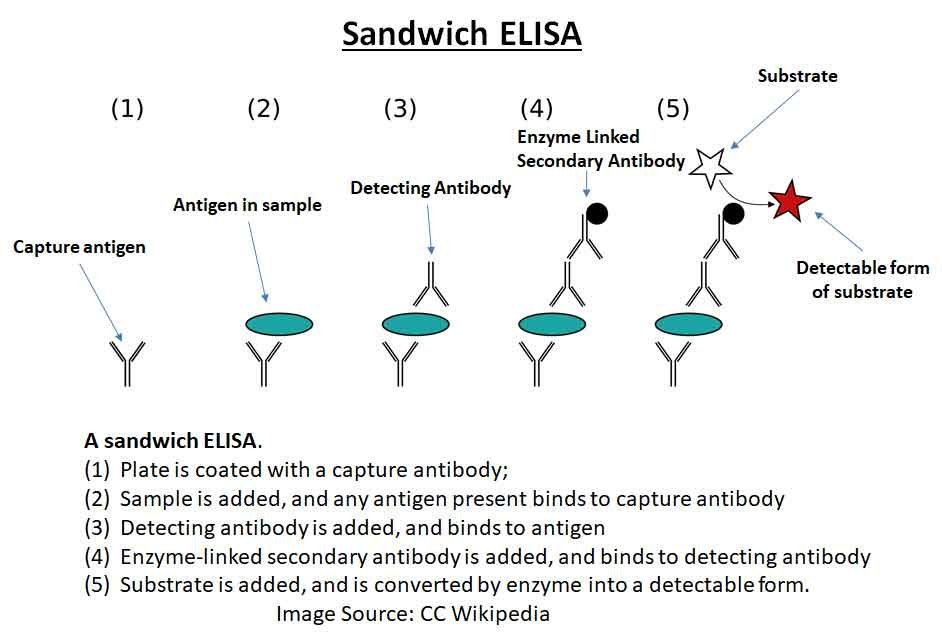
Sandwich ELISA:
Sandwich ELISA uses two antibodies—the first one captures the antigen and the second one, which is enzyme-linked, detects it.
In this method, the antigen is “sandwiched” between two layers of antibodies: a capture antibody that binds to the antigen and a detection antibody that binds to a different site on the antigen. The detection antibody is typically linked to an enzyme that, upon addition of a substrate, produces a measurable signal such as color change, fluorescence, or electrochemical change, indicating the presence and amount of the target antigen.
A “sandwich” ELISA is used to detect sample antigen. The steps are:
- A surface is prepared with a known amount of ‘capture’ antibody.
- Any nonspecific binding sites on the surface are blocked.
- The antigen-containing sample is applied to the plate and captured by the antibody.
- The plate is washed to remove any unbound antigen.
- A specific antibody is added, binding to the antigen (creating a “sandwich” with the antigen between two antibodies). This primary antibody could be in the serum of a
- donor being tested for reactivity towards the antigen.
- Enzyme-linked secondary antibodies are applied as detection antibodies, binding specifically to the Fc region of the primary antibody.
- The plate is washed to remove any unbound antibody-enzyme conjugates.
- A chemical is added that is converted by the enzyme into a color, fluorescent, or electrochemical signal.
- The absorbance, fluorescence, or electrochemical signal of the plate’s wells is measured to determine the presence and quantity of the antigen.
Commonly Used Enzymatic Markers used in ELISA Detection
Ø ABTS (2,2′-Azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt) turns green when detecting HRP.
Ø PNPP (p-Nitrophenyl Phosphate, Disodium Salt) turns yellow when detecting alkaline phosphatase enzyme
Ø OPD (o-phenylenediamine dihydrochloride) turns amber to detect HRP (horseradish peroxidase enzyme), which is often used to as a conjugated protein.
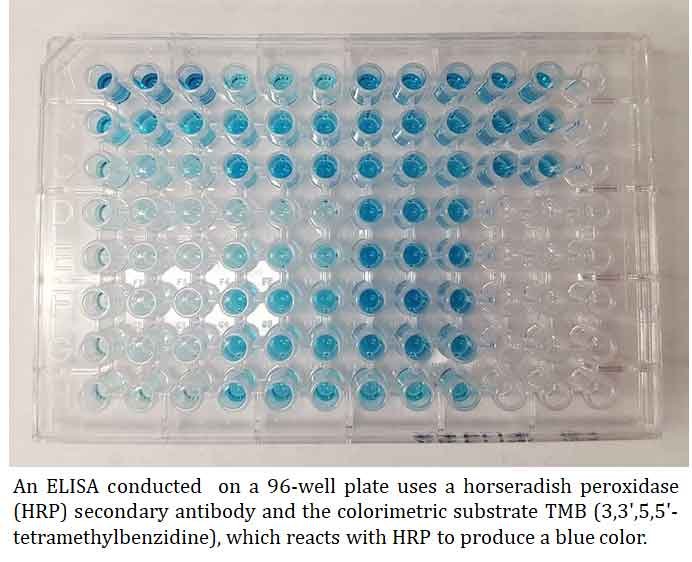
Ø MB (3,3′,5,5′-tetramethylbenzidine) turns blue when detecting HRP and turns yellow after the addition of sulfuric or phosphoric acid
Real-World Examples
Example 1: Detecting HIV
One of the most common uses of ELISA is in the detection of HIV (Human Immunodeficiency Virus) antibodies in blood samples. Here’s how it works:
Step 1: A plate is coated with HIV antigens.
Step 2: The patient’s blood sample is added. If HIV antibodies are present, they will bind to the antigens on the plate.
Step 3: An enzyme-linked secondary antibody, which binds to the HIV antibodies, is added.
Step 4: A substrate is added, and the enzyme reacts with it to produce a color change.
Step 5: The color intensity indicates the presence of HIV antibodies in the blood.
Example 2: Food Allergy Testing
ELISA is also used to detect allergens in food products, ensuring they are safe for people with allergies.
Step 1: Wells are coated with antibodies specific to the allergen (e.g., peanuts).
Step 2: A food sample extract is added. If the allergen is present, it will bind to the antibodies.
Step 3: A detection antibody, linked to an enzyme, is added.
Step 4: A substrate is added, producing a color change if the allergen is present.
Step 5: The color intensity indicates the amount of allergen in the food sample.
Want to read offline? download PDF: ELISA Short Notes
Why is ELISA Important?
ELISA is a crucial tool in both clinical and research settings because it is:
Highly Sensitive: Can detect minute amounts of antigens or antibodies.
Versatile: Used for a wide range of applications from disease diagnosis to food safety.
Provides Quantitative Results: Allows for the measurement of the concentration of the target molecule.
Conclusion
ELISA is like a molecular detective, helping scientists and doctors detect and measure important substances in biological samples. Whether it’s diagnosing a viral infection, testing for allergies, or ensuring food safety, ELISA plays a vital role in modern science and medicine.
<<< Back to Biotechnology Notes
I hope you found this article on ELISA Technique is informative and beneficial. Your feedback and comments would be greatly appreciated. Whether you have suggestions, questions, or thoughts to share, I would be delighted to hear from you. Engaging with your comments helps me continue to produce high-quality content in Biology. Please feel free to leave a comment below. Thank you for your support.
Regards: EasyBiologyClass
| You may also like NOTES in... | ||
|---|---|---|
| BOTANY | BIOCHEMISTRY | MOL. BIOLOGY |
| ZOOLOGY | MICROBIOLOGY | BIOSTATISTICS |
| ECOLOGY | IMMUNOLOGY | BIOTECHNOLOGY |
| GENETICS | EMBRYOLOGY | PHYSIOLOGY |
| EVOLUTION | BIOPHYSICS | BIOINFORMATICS |
